The Center for Advanced Medical Learning and Simulation (CAMLS) is dedicated to inspiring world-class medical education, training, and research that transform the delivery of healthcare services for the benefit of patients. Based in Tampa, Florida, CAMLS operates a 90,000-square-foot, state-of-the-art facility providing every possible form of health-professional education and training, including assistance with research studies and product development.
CAMLS integrates simulation technology, aviation science, team training, and evidence-based best practices into innovative programs with measurable outcomes. The organization’s Tampa Bay Research & Innovation Center (TBRIC) collaborates with physicians and medical device manufacturers by combining cutting-edge simulation technologies with research and innovation to move the latest advances in healthcare into practice.
To support its collaborative development programs, CAMLS needed an integrated 3D development platform with extensive design and simulation capabilities, according to Chief Engineer Mario Simoes. “Our mission is to work with physicians and manufacturers to accelerate development of innovative medical devices and procedures,” Simoes says. “To achieve our objectives, we need robust yet integrated design and simulation capabilities—ranging from structural and thermal analysis to fluid flow and mold-filling simulation—to streamline the development and accelerate the availability of new diagnostic equipment.”
CAMLS turned to Dassault Systèmes SolidWorks Corporation to support its unique development needs because the SOLIDWORKS family of products offers the software tools that CAMLS needs within the easiest-to-use and most tightly integrated platform. The organization utilizes SOLIDWORKS Premium design, SOLIDWORKS Simulation Premium analysis, SOLIDWORKS Flow Simulation computational fluid dynamics (CFD) analysis (including its Electronics Cooling Module), SOLIDWORKS Plastics mold-filling analysis, SOLIDWORKS Enterprise PDM product data management, SOLIDWORKS Sustainability Environmental impact assessment, and SOLIDWORKS Composer™ technical communication software.
“To speed time-to-market, we need to streamline the process for designing, validating, and manufacturing a medical device,” Simoes notes. “Because SOLIDWORKS provides a fully integrated suite of design, simulation, data management, and communication tools, we believed it would best enable us to consistently achieve that goal.”
COMBINING TWO PROCEDURES INTO ONES
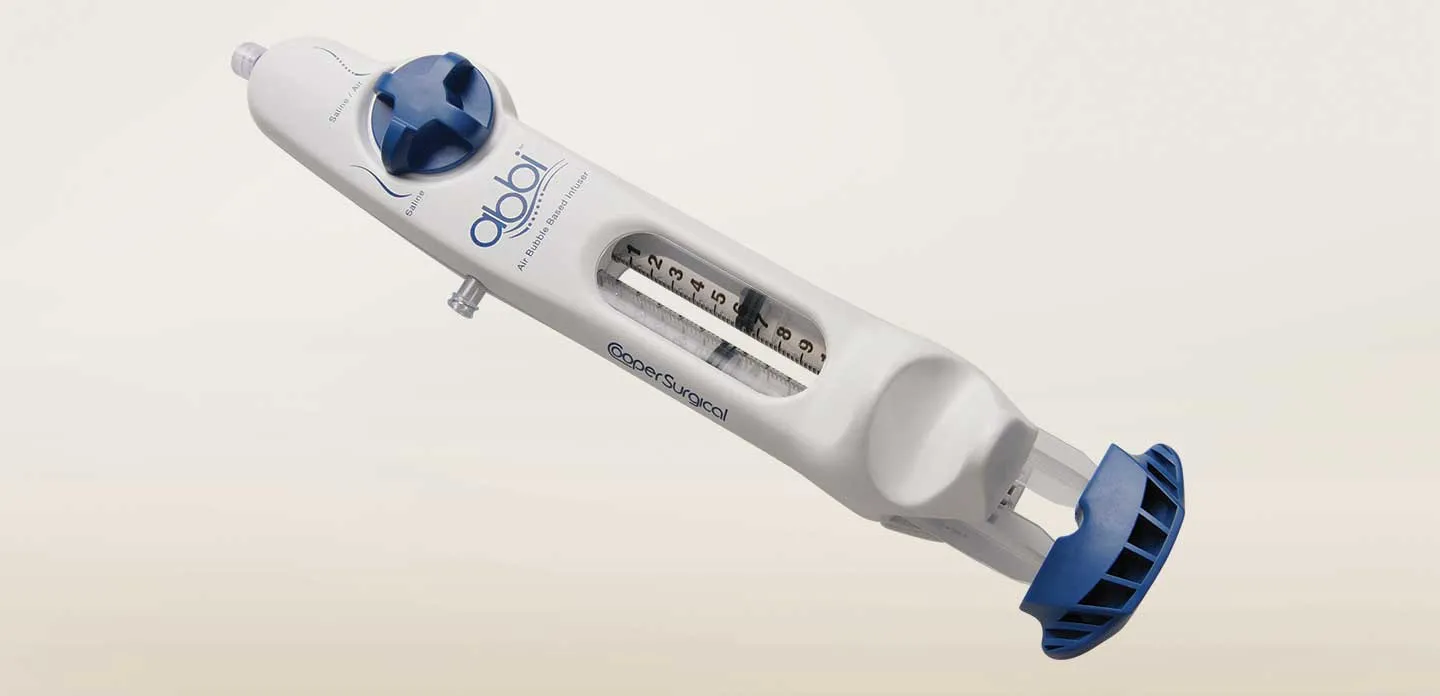
TBRIC used SOLIDWORKS when working with CooperSurgical, Inc. on the development of a new device for conducting sonohysterosalpingography (sono-HSG), an ultrasound exam for studying the contour of the uterine cavity and the patency of the fallopian tubes to determine potential fertility issues.
“Using SOLIDWORKS, we were able to cut development time by 30 percent,” Simoes stresses. “Medical device development takes more time than designing other types of products because we have to validate every step, both in software and through the production of prototypes to support usability studies to comply with U.S. Food and Drug Administration [FDA] requirements. The combination of integrated SOLIDWORKS tools and the ability to conduct testing all within the same facility shortened the process and accelerated time-to-market.”
ABBI (Air Bubble Based Infuser) is a single-use device that facilitates two procedures as part of an initial female fertility evaluation: a sono-HSG for tubal patency and an SIS for uterine structure. Both are office-based procedures using existing ultrasound systems and an SIS catheter. These procedures can be performed in an IVF center or OB/GYN practice that offer fertility screening.